
New Indication Added! Jiangsu Vcare’s Second-Generation Highly Selective JAK1 Inhibitor VC005 Tablets Approved for IND in Oral Treatment of Vitiligo
Published Time:
2025-05-09 17:38
Source:
On May 8, 2025, Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) achieved another major milestone in its self-developed second-generation highly selective JAK1 inhibitor, VC005 tablets. The project, intended for the oral treatment of non-segmental vitiligo received official IND approval from the NMPA (Application No.: CXHL2500200).
The VC005 project demonstrates picomolar-level inhibition of JAK1 in vitro, providing a potent mechanism for reversing vitiligo progression. Whigh selectivity towards JAK1 than JAK2, it is expected to mitigate hematological side effects caused by excessive JAK2 inhibition. Additionally, VC005’s inhibitory effects on JAK3 and TYK2 synergize to enhance efficacy, particularly by significantly suppressing the IL-15 – JAK1/3 signaling pathway associated with vitiligo recurrence. This positions VC005 to deliver superior long-term efficacy in delaying disease relapse.
Currently, the VC005 tablet is in Phase III clinical trial in China, while the co-developed VC005 topical gel has entered Phase II clinical trial. This IND approval marks the addition of vitiligo as a new indication to VC005’s clinical development pipeline, following its ongoing trials for moderate-to-severe atopic dermatitis and ankylosing spondylitis. The company plans to initiate a Phase II clinical study for this new indication shortly.
About VC005
VC005 is a novel, potent, and highly selective next-generation JAK1 inhibitor independently developed by Jiangsu Vcare. By selectively inhibiting JAK1, the drug reduces inflammatory responses and immune cell activation, making it clinically applicable for treating inflammatory and autoimmune diseases. It is available in two formulations: oral tablets and topical gel.
The oral tablet was developed for multiple autoimmune conditions, including moderate-to-severe atopic dermatitis, ankylosing spondylitis, rheumatoid arthritis, and vitiligo, with the most advanced progress in Phase III trials for moderate-to-severe atopic dermatitis. The topical gel, targeting mild-to-moderate atopic dermatitis, is currently in Phase II trial. Compared to Upadacitinib, a leading marketed drug in the same class, VC005 more selectively reduces JAK2 inhibitory activity (based on in vitro kinase assay results), potentially alleviating safety concerns associated with excessive JAK2 inhibition in clinical settings.
About Vitiligo
Vitiligo is a localized or generalized depigmentary disorder caused by a reduction or disappearance of tyrosinase activity in melanocytes within the skin and hair follicles, resulting in decreased or loss of melanin granule production. It is characterized by complete loss of skin and mucous membrane pigmentation in a localized or generalized manner. It can occur in any part of the body, commonly on the back of the fingers, wrists, forearms, face, neck, etc.
The worldwide lifetime prevalence was estimated at 0.36% in the general population, affecting an estimated 28.5 million people worldwide.
The target mechanism of VC005 is well-defined. In patients with vitiligo, it works in two ways:
By inhibiting the IFN-γ - JAK1/2 - STAT1 signaling pathway, it blocks keratinocytes from releasing chemokines that recruit more CD8+ T cells.
By inhibiting the IL-15 - JAK1/3 - STAT3/5 signaling pathway, it suppresses the activation of Trm cells.
This is how VC005 aims to achieve disease reversal and delay recurrence.
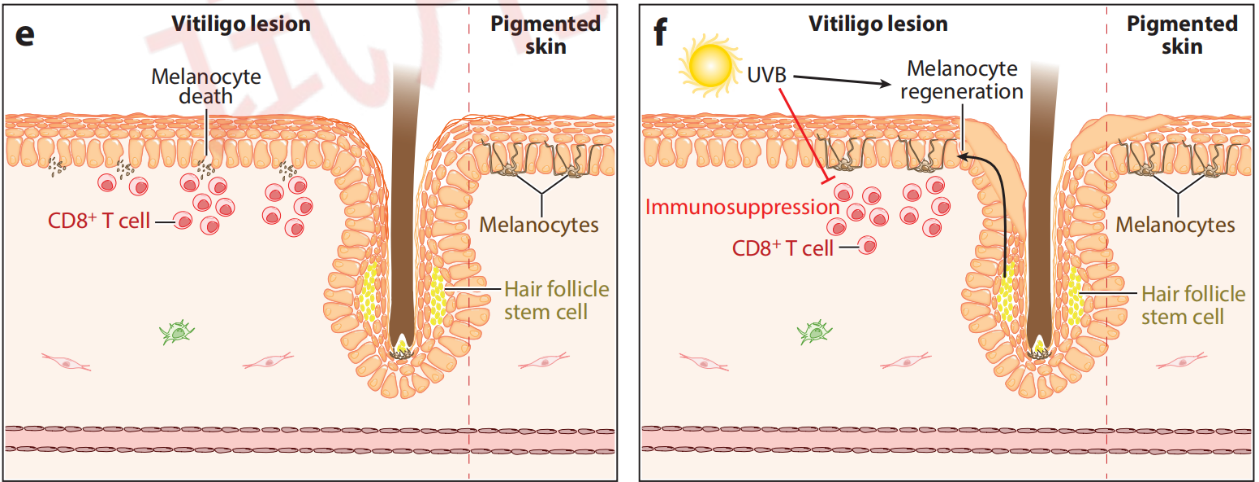
Vitiligo Pathogenesis and Therapeutic Mechanisms
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
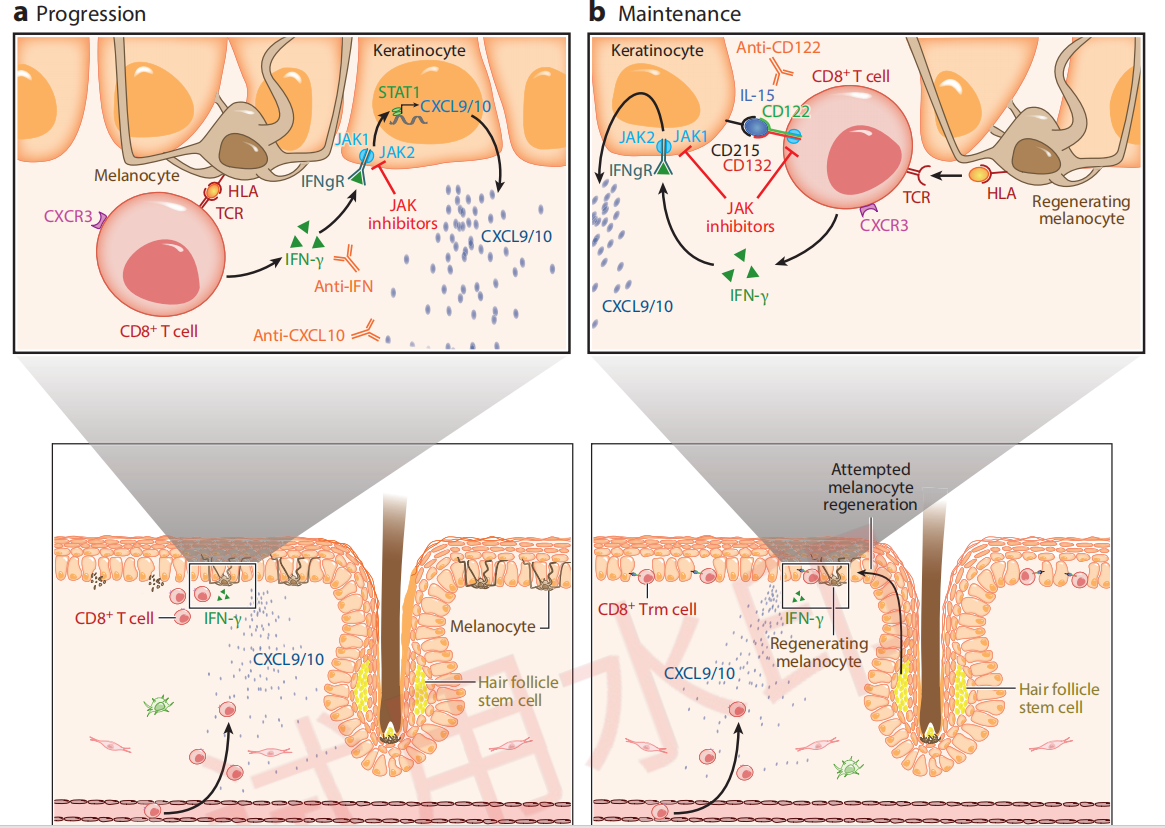
Immunological Mechanisms of Vitiligo Progression and Maintenance
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
Previous Page
Related News
27
2017
/
03
Vice Chairman of Political Consultative Conference of Wenzhou Ouhai District Visited Jiangsu Vcare
The delegation of Political Consultative Conference of Wenzhou Ouhai District, accompanied by vice chairman of Political Consultative Conference of Pukou Economic Development Zone visited Jiangsu Vcare Pharmaceutical Technology Co., Ltd. on the afternoon of March 27, 2017. Yongqiang Liu, the general manager of Jiangsu Vcare received the delegation. Mr. Liu introduced the company's development history and R&D progress. He focused on the progress of phase II clinical trials and the future market prospect of innovative drug Vicagrel. The delegation highly recognized Vcare's advanced equipment and facilities, harmonious human environment and comprehensive research and development capabilities.
14
2017
/
03
Jiangsu Vcare Held “Firefighting and First Aid” Training
Jiangsu Vcare invited Lei Wang, a special instructor of Nanjing Anqu Fire Education Center to give a “Firefighting and First Aid” course to new employees on March 14, 2017 and 26 new employees participated in the training. Mr. Wang collected a large number of fire cases and carefully analyzed the causes of these fires. He taught everyone how to deal with fires. His explanations made everyone fully aware of the importance and urgency of fire safety. At the same time, everyone had a better understanding of various fire extinguishers and their instructions.

