
New Indication Added! Jiangsu Vcare’s Second-Generation Highly Selective JAK1 Inhibitor VC005 Tablets Approved for IND in Oral Treatment of Vitiligo
Published Time:
2025-05-09 17:38
Source:
On May 8, 2025, Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) achieved another major milestone in its self-developed second-generation highly selective JAK1 inhibitor, VC005 tablets. The project, intended for the oral treatment of non-segmental vitiligo received official IND approval from the NMPA (Application No.: CXHL2500200).
The VC005 project demonstrates picomolar-level inhibition of JAK1 in vitro, providing a potent mechanism for reversing vitiligo progression. Whigh selectivity towards JAK1 than JAK2, it is expected to mitigate hematological side effects caused by excessive JAK2 inhibition. Additionally, VC005’s inhibitory effects on JAK3 and TYK2 synergize to enhance efficacy, particularly by significantly suppressing the IL-15 – JAK1/3 signaling pathway associated with vitiligo recurrence. This positions VC005 to deliver superior long-term efficacy in delaying disease relapse.
Currently, the VC005 tablet is in Phase III clinical trial in China, while the co-developed VC005 topical gel has entered Phase II clinical trial. This IND approval marks the addition of vitiligo as a new indication to VC005’s clinical development pipeline, following its ongoing trials for moderate-to-severe atopic dermatitis and ankylosing spondylitis. The company plans to initiate a Phase II clinical study for this new indication shortly.
About VC005
VC005 is a novel, potent, and highly selective next-generation JAK1 inhibitor independently developed by Jiangsu Vcare. By selectively inhibiting JAK1, the drug reduces inflammatory responses and immune cell activation, making it clinically applicable for treating inflammatory and autoimmune diseases. It is available in two formulations: oral tablets and topical gel.
The oral tablet was developed for multiple autoimmune conditions, including moderate-to-severe atopic dermatitis, ankylosing spondylitis, rheumatoid arthritis, and vitiligo, with the most advanced progress in Phase III trials for moderate-to-severe atopic dermatitis. The topical gel, targeting mild-to-moderate atopic dermatitis, is currently in Phase II trial. Compared to Upadacitinib, a leading marketed drug in the same class, VC005 more selectively reduces JAK2 inhibitory activity (based on in vitro kinase assay results), potentially alleviating safety concerns associated with excessive JAK2 inhibition in clinical settings.
About Vitiligo
Vitiligo is a localized or generalized depigmentary disorder caused by a reduction or disappearance of tyrosinase activity in melanocytes within the skin and hair follicles, resulting in decreased or loss of melanin granule production. It is characterized by complete loss of skin and mucous membrane pigmentation in a localized or generalized manner. It can occur in any part of the body, commonly on the back of the fingers, wrists, forearms, face, neck, etc.
The worldwide lifetime prevalence was estimated at 0.36% in the general population, affecting an estimated 28.5 million people worldwide.
The target mechanism of VC005 is well-defined. In patients with vitiligo, it works in two ways:
By inhibiting the IFN-γ - JAK1/2 - STAT1 signaling pathway, it blocks keratinocytes from releasing chemokines that recruit more CD8+ T cells.
By inhibiting the IL-15 - JAK1/3 - STAT3/5 signaling pathway, it suppresses the activation of Trm cells.
This is how VC005 aims to achieve disease reversal and delay recurrence.
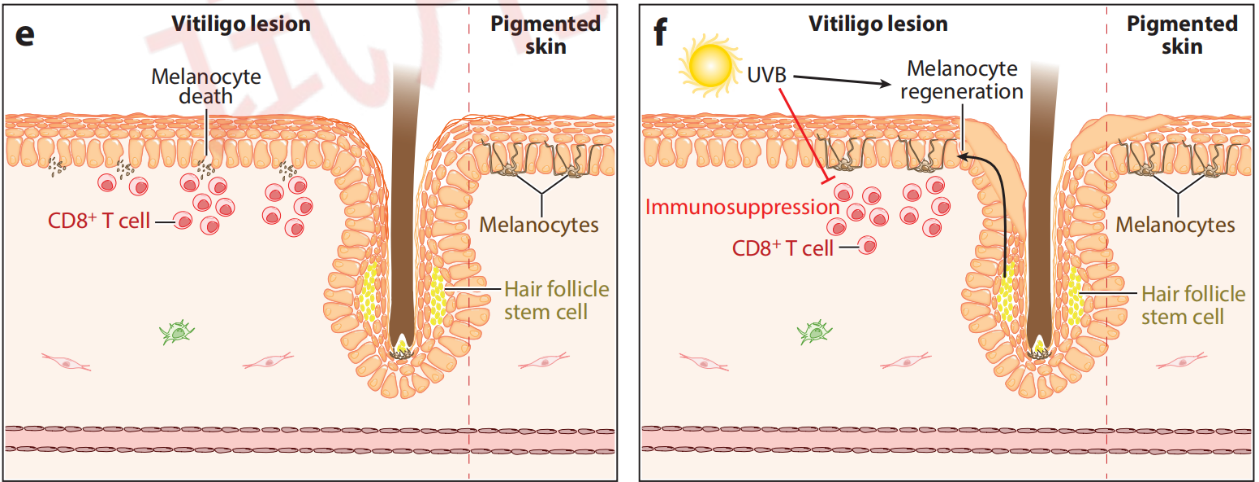
Vitiligo Pathogenesis and Therapeutic Mechanisms
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
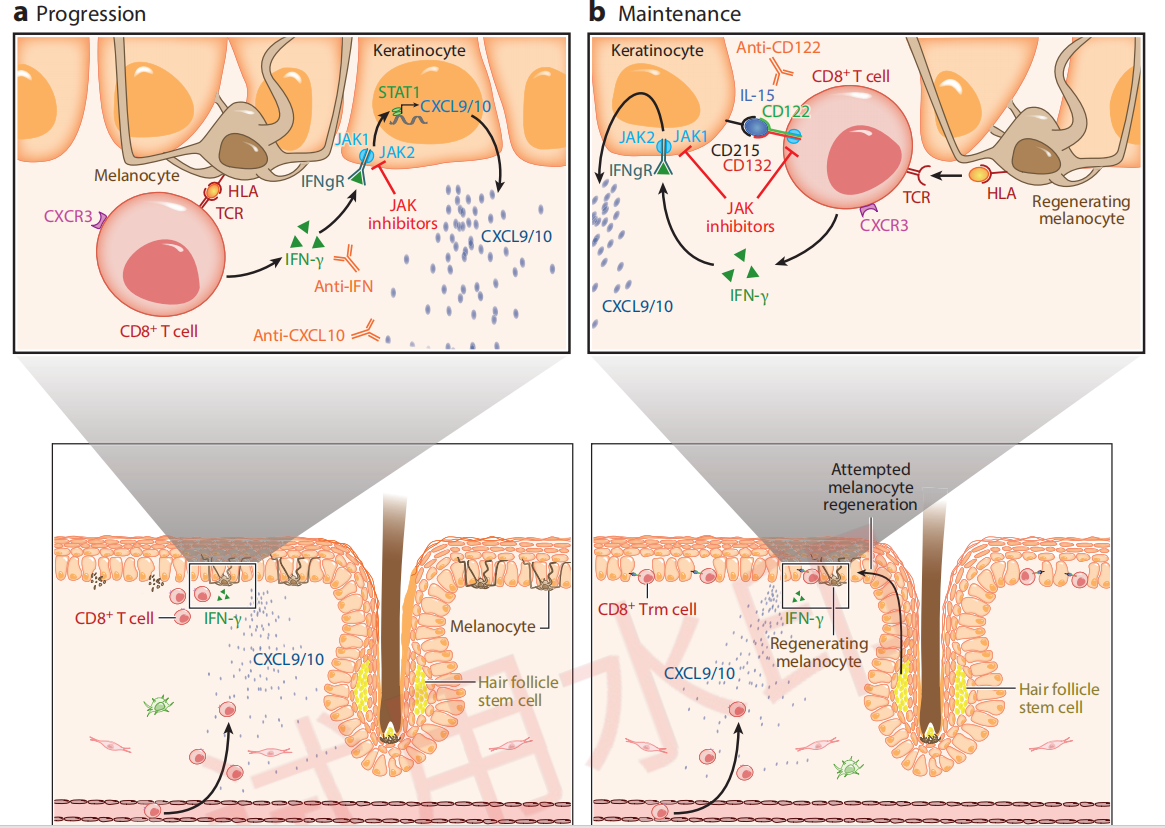
Immunological Mechanisms of Vitiligo Progression and Maintenance
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
Previous Page
Related News
16
2021
/
08
Jiangsu Vcare's Class 1 Innovative Drug VC005 Tablets Receives Clinical Trial Approval
In August 2021, VC005 tablets, an innovative drug developed by Jiangsu Vcare PharmaTech Co., Ltd. (Jiangsu Vcare), received clinical trial approval from the NMPA for the indication of inflammatory bowel disease (IBD), with clinical trials set to begin shortly.
29
2021
/
07
Jiangsu Vcare Secures New Round of Financing Totaling CNY 100 Million
Recently, Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) secures another strategic investment of CNY 100 million. This round was jointly invested by Nanjing NII Group, Renpu Capital, and Nanjing Innovation Capital Group. The funds will primarily be used to advance the clinical research of innovative drug pipeline projects like Vicagrel and VC004, while accelerating strategic layout in the CDMO business sector.
24
2021
/
06
Vcare Pharmatech Selected as 2021 Nanjing Nurtured Unicorn Enterprise
On June 23, 2021, the list of Nanjing Unicorn and Gazelle Enterprises was announced. Jiangsu Vcare Pharmatech Technology Co., Ltd.(Vcare Pharmatech) was selected as a 2021 Nanjing Nurtured Unicorn Enterprise.
22
2021
/
03
Jiangsu Vcare's Novel Drug Vicagrel Receives FDA Approval for Clinical Trials
Recently, Jiangsu Vcare PharmaTech Co., Ltd. (Jiangsu Vcare) announced encouraging news regarding its novel antiplatelet drug Vicagrel: The US FDA has provided feedback on the company's submitted IND application for Vicagrel, permitting the company to initiate clinical trials.
12
2021
/
03
Jiangsu Vcare Completes Pre-IND Submission for VC005, a Highly Selective JAK1 Inhibitor New Drug
Recently, Jiangsu Vcare PharmaTech Co., Ltd. (Jiangsu Vcare) successfully submitted a Pre-IND application to the NMPA for its VC005 tablets. VC005 is a highly selective JAK1 inhibitor independently designed by Jiangsu Vcare. It is intended for the clinical treatment of inflammatory bowel disease (IBD), an intestinal autoimmune disorder.
08
2021
/
03
Jiangsu Vcare Closes Hundreds-of-Millions-of-RMB Financing Round
Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) has secured financing worth hundreds of millions of CNY in a round led by SDIC Capital, with participation from Fengling Capital. The funds will accelerate Vcare’s CDMO expansion and Phase III clinical research for its innovative drug Vicagrel.

