
New Indication Added! Jiangsu Vcare’s Second-Generation Highly Selective JAK1 Inhibitor VC005 Tablets Approved for IND in Oral Treatment of Vitiligo
Published Time:
2025-05-09 17:38
Source:
On May 8, 2025, Jiangsu Vcare Pharmatech Co., Ltd. (Jiangsu Vcare) achieved another major milestone in its self-developed second-generation highly selective JAK1 inhibitor, VC005 tablets. The project, intended for the oral treatment of non-segmental vitiligo received official IND approval from the NMPA (Application No.: CXHL2500200).
The VC005 project demonstrates picomolar-level inhibition of JAK1 in vitro, providing a potent mechanism for reversing vitiligo progression. Whigh selectivity towards JAK1 than JAK2, it is expected to mitigate hematological side effects caused by excessive JAK2 inhibition. Additionally, VC005’s inhibitory effects on JAK3 and TYK2 synergize to enhance efficacy, particularly by significantly suppressing the IL-15 – JAK1/3 signaling pathway associated with vitiligo recurrence. This positions VC005 to deliver superior long-term efficacy in delaying disease relapse.
Currently, the VC005 tablet is in Phase III clinical trial in China, while the co-developed VC005 topical gel has entered Phase II clinical trial. This IND approval marks the addition of vitiligo as a new indication to VC005’s clinical development pipeline, following its ongoing trials for moderate-to-severe atopic dermatitis and ankylosing spondylitis. The company plans to initiate a Phase II clinical study for this new indication shortly.
About VC005
VC005 is a novel, potent, and highly selective next-generation JAK1 inhibitor independently developed by Jiangsu Vcare. By selectively inhibiting JAK1, the drug reduces inflammatory responses and immune cell activation, making it clinically applicable for treating inflammatory and autoimmune diseases. It is available in two formulations: oral tablets and topical gel.
The oral tablet was developed for multiple autoimmune conditions, including moderate-to-severe atopic dermatitis, ankylosing spondylitis, rheumatoid arthritis, and vitiligo, with the most advanced progress in Phase III trials for moderate-to-severe atopic dermatitis. The topical gel, targeting mild-to-moderate atopic dermatitis, is currently in Phase II trial. Compared to Upadacitinib, a leading marketed drug in the same class, VC005 more selectively reduces JAK2 inhibitory activity (based on in vitro kinase assay results), potentially alleviating safety concerns associated with excessive JAK2 inhibition in clinical settings.
About Vitiligo
Vitiligo is a localized or generalized depigmentary disorder caused by a reduction or disappearance of tyrosinase activity in melanocytes within the skin and hair follicles, resulting in decreased or loss of melanin granule production. It is characterized by complete loss of skin and mucous membrane pigmentation in a localized or generalized manner. It can occur in any part of the body, commonly on the back of the fingers, wrists, forearms, face, neck, etc.
The worldwide lifetime prevalence was estimated at 0.36% in the general population, affecting an estimated 28.5 million people worldwide.
The target mechanism of VC005 is well-defined. In patients with vitiligo, it works in two ways:
By inhibiting the IFN-γ - JAK1/2 - STAT1 signaling pathway, it blocks keratinocytes from releasing chemokines that recruit more CD8+ T cells.
By inhibiting the IL-15 - JAK1/3 - STAT3/5 signaling pathway, it suppresses the activation of Trm cells.
This is how VC005 aims to achieve disease reversal and delay recurrence.
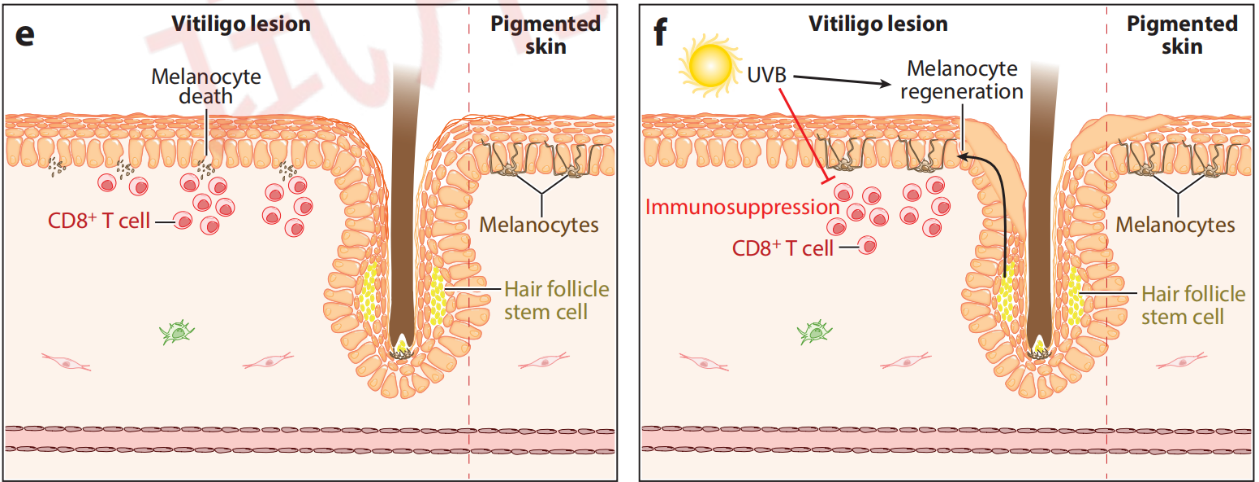
Vitiligo Pathogenesis and Therapeutic Mechanisms
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
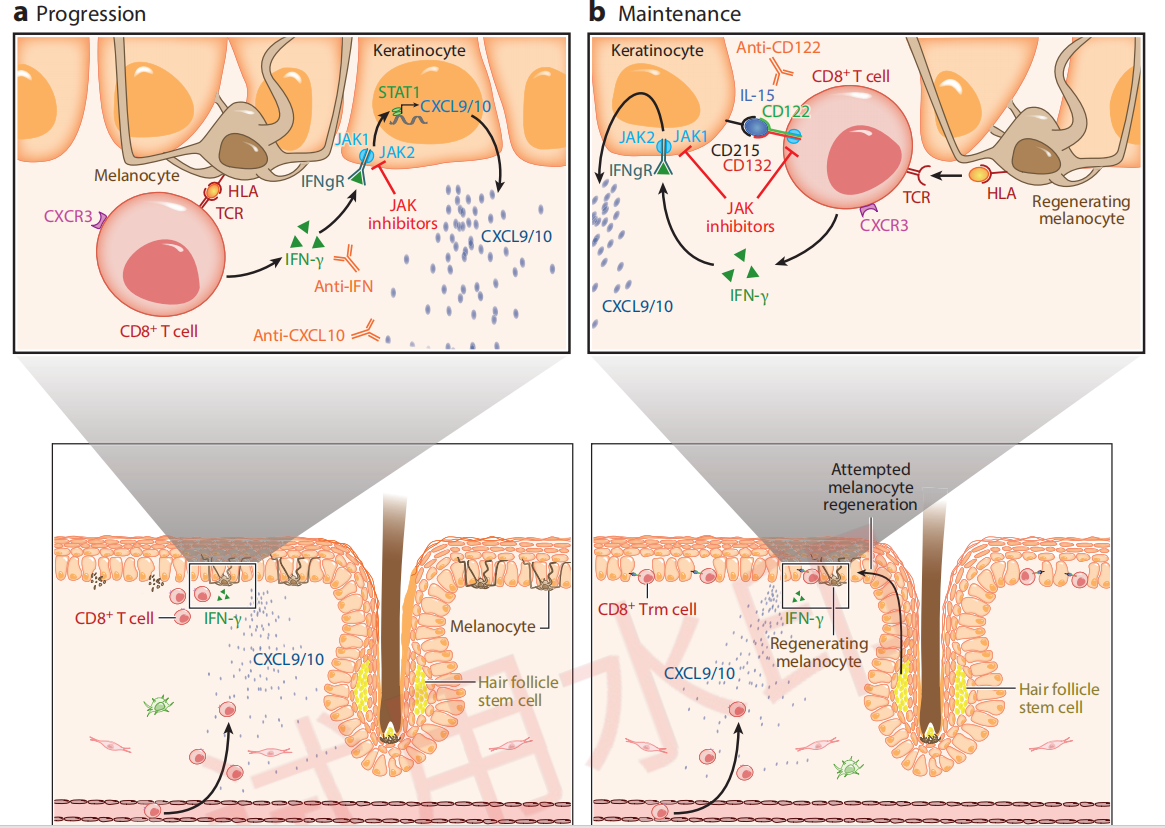
Immunological Mechanisms of Vitiligo Progression and Maintenance
Citing from Frisoli, et al, Vitiligo: Mechanisms of Pathogenesis and Treatment,2020
Previous Page
Related News
23
2017
/
08
Jiangsu Vcare Was Honored as Jiangbei New Area Lingque Enterprise
In 2017, the list of “Lingque” enterprises in Jiangbei New District of Nanjing was announced and Jiangsu Vcare Pharmaceutical Technology Co., Ltd. was successfully selected into the list. “Lingque” enterprises project aim to select a group of innovative SMEs with high growth in Jiangbei district and provide the selected enterprises with one-stop support such as equity investment, emergency turnover, loan interest subsidy, R&D subsidies, and etc.. To be successfully selected into the “Lingque” enterprises program is an inspiration to the company. Vcare keep integrating various resources to quickly become bigger and stronger to contribute to the development of Jiangbei New Area.
15
2017
/
06
Jiangsu Vcare and Yangzhou Princechem Acquired Boya Chemical Company
The signing ceremony of Jiangsu Vcare Pharmaceutical Technology Co., Ltd. and Yangzhou Princechem Co., Ltd. acquiring Boya Chemical (Nantong) Co., Ltd. was held in the conference room of Jiangsu Vcare recently. Boya Chemical (Nantong) Co., Ltd. is located in Yangkou Chemical Industrial Park, Rudong Coastal Economic Development Zone, Jiangsu Province. The company covers an area of 70 acres and is a Hong Kong-owned enterprise. The goal of this acquisition is to build Boya Chemical into a raw material drug manufacturer that meets the standards of China, the United States and the European Union. After renovation, Boya Chemical will be a chemical raw materials production base of Yatai Group and solve the bottleneck problem of Vcare's raw material drug R&D and production. It’s believed that the acquisition will make a significant contribution to Vcare’s production and sales of intermediates and APIs.
06
2017
/
06
Leaders of Management Committee of Rudong Coastal Economic Development Zone Visited Jiangsu Vcare
Leaders of management committee of Rudong coastal economic development zone visited Jiangsu Vcare on June 1st, 2017. The delegation was received by the vice chairman Jun Qu, general manager Yongqiang Liu, and vice general manager Yanchun Gong of Vcare. The delegation visited the Vcare's chemical research center and drug research center. Mr. Liu introduced R&D progress and advanced equipment to the guests. During the discussion, Mr. Liu introduced the overall development plan of Yatai Group's pharmaceutical industry, and Vcare's development history and the future plan. Mr. Liu also introduced the progress of innovative drug Vicagrel and a series of generic drugs in detail. The leaders of Rudong coastal economic development zone introduced the planning of their park, local investment, environmental protection policies and etc., and expressed their confidence in the research and development prospects of Vcare.
15
2017
/
05
Leaders of Pukou District Economic and Trade Bureau Visited Jiangsu Vcare
Leaders of Pukou District Economic and Trade Bureau Visited Jiangsu Vcare on May 12, 2017. The general manager Yongqiang Liu received the delegation.
24
2017
/
04
Jiangsu Vcare Held Mountain Climbing and Tug-of-war Competitions
Jiangsu Vcare organized mountain climbing and tug-of-war activities recently. The mountain climbing competition was divided into men's and women's groups, and each group competed for top three. The venue was located in Laoshan National Forest Park. Everyone climbed the top after efforts. Four teams consisting of Vcare’s two R&D center members conducted a tug-of-war competition after going down the mountain and finally "Lingya team" won the top spot. Everyone had fun.
30
2017
/
03
Jiangsu Vcare Held the First Quarter Fire Drill
In order to further improve the safety awareness of new employees, Jiangsu Vcare held a fire-fighting exercise on March 30, 2017. New employees had a deeper understanding of fire extinguishers through on-site explanation of the types, inspection methods and instructions of fire extinguishers. Everyone learned about fire-fighting procedures and the use of appliances. The exercise provided a safety guarantee for everyone’s life and work.

